WATCH THE VIDEO HERE
OR READ ON BELOW!
- Elemental sulphur attack is NOT sulphidation or hydrogen sulphide corrosion.
- It is an aqueous corrosion phenomenon.
- It considers two modes -
- acidification of sulphur - formation of sulphuric acid
- The lowering of pH is the main source of corrosion in both the methods.
- The phenomenon is temperature dependent. It increases with increase in temperature and becomes particularly severe above the melting point of sulphur (~112.8 degree Celsius).
- Hydrogen sulphide present in the petroleum may aggravate the sulphur attack by enhancing uniform pitting corrosion.
- Monoethylene glycol is used to prevent condensate formation ans may be present in traces in the feedstock petroleum. This enhances the sulphur attack in the form of uniform corrosion, and crevice corrosion.
References:
- Fang, Haitao, Brown, Bruce, Young, David, and Srdjan Nešic. "Investigation Of Elemental Sulfur Corrosion Mechanisms." Paper presented at the CORROSION 2011, Houston, Texas, March 2011.
- Yoon, Yuhchae, Srinivasan, Sridhar, Yap, Kwei-Meng, and Russell D. Kane. "Elemental Sulfur and Speciation in High Pressure High Temperatures Oil and Gas Well Environments: Their Role in Stress Corrosion Cracking of Corrosion Resistant Alloys." Paper presented at the CORROSION 2017, New Orleans, Louisiana, USA, March 2017.
- Yaakob, Nurul & Singer, M. & Young, David. (2015). Elemental sulfur corrosion of carbon steel in the presence of sulfur solvent and monoethylene glycol. NACE - International Corrosion Conference Series. 2015.
😀Happy learning!😀


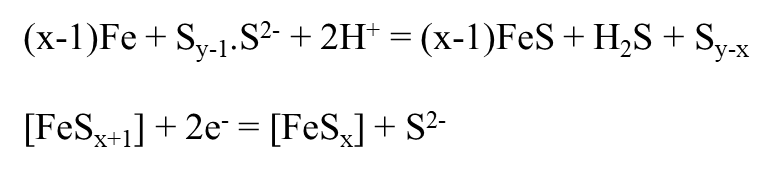
Comments
Post a Comment
Welcome to the CORROSPECTIVE BLOG! Scroll along for more posts! Looking forward to your feedback!
P.S. Don't forget to check out the free quizzes!